CAR T-Cell Therapy Program
Northwestern Medicine offers CAR T-Cell Therapy through the Robert H. Lurie Comprehensive Cancer Center of Northwestern University and Northwestern Memorial Hospital for patients with blood cancers (leukemia, lymphoma, myeloma) and potentially other types of cancers being studied in clinical trials.
What is CAR T-cell therapy?
CAR T-cell therapy is a form of immunotherapy that involves modifying your immune system to fight certain types of cancer. CAR T-cell therapy is available at Northwestern Medicine through standard of care treatments that have been approved by the FDA and through clinical trials.
How does it work?
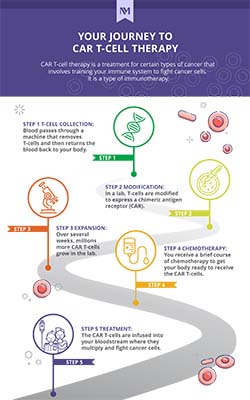
The process begins with the collection of T-cells (a type of white blood cell) from your blood through a large vein during a procedure called leukapheresis. The collected T-cells are transported to a laboratory where they are genetically modified to express a specific protein on the surface of the cell called a chimeric antigen receptor (CAR). The modified T-cells are then reinfused into your body where the new receptors help them to recognize and destroy cancer cells.
What are the potential side-effects?
CAR T-cell therapy is a highly unique treatment that is associated with side effects that can be severe and even life-threatening. It may cause side effects that can be severe and even life-threatening. The CAR T-cell therapy care team at Northwestern Medicine is specially trained in identifying and treating these side effects. The most frequent side effects are described below.
- Cytokine Release Syndrome (CRS): In some patients, the immune system may become overactive as the CAR T-cells travel through the body. This causes substances called cytokines to release into the system. This can make you feel like you have the flu, with a high fever and/or chills. Other symptoms that may occur include low blood pressure, difficulty breathing, or confusion. These symptoms can be mild or severe. Your team will monitor you frequently to help control these symptoms.
- Neurologic side effects: In some patients, the immune activation after CAR T-cell infusion may alter the brain and neurologic system temporarily. These changes can present as confusion, difficulty with talking or memory, or even in severe cases, loss of consciousness. Your team will monitor you frequently and may give you special medications to help prevent or control these problems.
If you qualify for CAR T-cell therapy, your care team at Northwestern Medicine will talk with you in more detail about these and other side effects.
Who can benefit from CAR T-cell therapy?
CAR T-cell therapy has been approved by the U.S. Food and Drug Administration to treat:
- Patients with some forms of fast-growing, non-Hodgkin lymphoma that has progressed after prior treatment(s)
- Patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL)
- Patients with multiple myeloma that has progressed after prior treatment(s)
CAR T-cell therapy is also currently being studied in clinical trials for its effectiveness with other types of cancers. Click below to find CAR T-cell therapy clinical trials at Northwestern Medicine.
Meet the Team

The CAR T-Cell Therapy Program includes Northwestern Medicine physicians, working with the Robert H. Lurie Comprehensive Cancer Center of Northwestern University and Northwestern Memorial Hospital.
Why Choose Northwestern Medicine
The Robert H. Lurie Comprehensive Cancer Center of Northwestern University at Northwestern Memorial Hospital is a leader in cancer care.
- The Lurie Cancer Center is the only Comprehensive Cancer Center in Illinois to receive an “Exceptional” rating from the National Cancer Institute – the largest funder of cancer research in the world.
- The Lurie Cancer Center is one of 28 members of the National Comprehensive Cancer Network and the only cancer center member in Illinois.
- Treatment is provided at Northwestern Memorial Hospital and Robert H. Lurie Comprehensive Cancer Center of Northwestern University at Northwestern Memorial Hospital is proud to be a nationally recognized cancer program by U.S. News & World Report.
- The Lurie Cancer Center has been conducting CAR T-cell therapy clinical trials since 2016.
- Northwestern Memorial Hospital has two unique specialty centers on the downtown campus that also support the care of our CAR T-cell patients:
- The Rube Walker Blood Center (RWBC): Northwestern Medicine's outpatient blood center is a place where patients can receive a variety of blood therapies such as transfusions, apheresis and IVIG. RWBC is the only location at Northwestern Medicine that performs cell collection for standard of care treatments and clinical trials across a broad range of specialties, including behavioral health, cancer care, digestive health, cardiovascular care, neurosciences, rheumatology, solid organ transplant and women's health.
- The Mathews Center for Cellular Therapy (MCCT): Northwestern Medicine has a cellular therapy laboratory that offers researchers, clinicians, and biotechnology companies regulatory compliant labs and services to manufacture more than minimally manipulated and complex biotechnology products at the downtown campus. MCCT has four product manufacturing suites that follow good manufacturing practices (GMP) and good tissue practices (GTP) and are adaptable to most biologic and cellular processes for phase I to III clinical trials. They also offer a large general lab meeting GTP and good laboratory practices (GLP).